In (Finding chemical structures – InChIs et al., an amusement) I explored the varied approaches to drawing structures and the problems of representing them. I commented that Totally Synthetic’s diagrams were not only the most unambiguous but also the most beautiful. I now regret having done that, as Steve Bachrach has argued that this makes them copyrightable.
PMR; Steve is a strong evangelist for OA and we are on the same side but I don’t think arguing for copyrighting chemical structure diagrams is helpful. Let’s take the analogy of mathematical equations. I could argue that
- (taken from Wikipedia on Euler) was beautiful, while
- ex = Σ(&infty;n=0)xn/n! was ugly.
Let’s assume that Euler had access to TeX and had published his formula in a journal belonging to the strong-copyright school of thought and that a student has cut and pasted the formula to explain the summation. The publisher could then claim “the formula is beautifully typeset so you must take it down, or retype it”. Poor Euler is dead so hasn’t any say.
In the same way the chemical formula diagram is the ONLY means of communicating the structure. In TotSynth’s case the wedge bonds in ring B are not to make it pretty but to emphasize what the compound actually is

while the PNAS structure tries to do the same but in a much uglier and gritty fashion. There is a real likelihood of confusion as to what the structure actually is:
-
DrZZ Says:
September 24th, 2007 at 12:26 pm eInteresting stuff. Let me add some additional points. One of the structures in PubChem comes from us (DTP/NCI). If you look at the compound record you get the mess you included above. If you navigate to the substance record (click on the CID, on that page look for the Substance: 1 link, when that hit comes up click on the SID, and on that page change the drop down choice for Compound Displayed from PubChem to deposited) you see a much more sensible 2D drawing. In a quick look, I think the difference between the two structures in PubChem is that one of the stereocenters in the NCI deposited structure is unspecified in the other structure. The NCI structure was submitted in 1997 by one of the authors of the original isolation paper. As the structure correction was published in 2001, it is almost certain that the NCI structure contains the original error. I say almost because we have no audit trail in our internal database for structures (at least not easily visible to me). A NSC has a structure, period. There is some possibility that the structure was fixed, but that just overwrites the previous structure. It just reinforces my view that it is extremely important to treat the structure of a substance as one more data point, subject to varied and possibly conflicting values.

It’s very easy to get it wrong.
Now if I cut and paste the diagrams and say “this one shows clearly that the ring is sticking up” I might help avoid the wrong compound being given to the wrong patient somewhere down the line. (This is not hypothetical – these are possible drugs). Steve, can you justify a publisher saying – “we’ll send the lawyers after you for posting copyright chemical structures”?
Because if so, the C21 will be enormously impoverished. So yes, it’s beautiful. but NO it mustn’t be copyrighted by publishers.
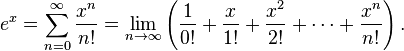
September 24th, 2007 at 5:23 pm eThis discussion demonstrates why I believe that “structure drawing” falls within the domain of materials than can be copyrighted. What is the difference in all of these representations (let’s agree not to worry about what might be a different stereochemistry at one center)? In the eye of the viewer some of these are “ugly” and some are more aesthetically pleasing. I might argue that Totally Synthetic’s representation is not only clear, it might even be called “pleasing”.Since it is aesthetics (beauty, clarity, etc) that differentiates these drawings, the creator’s choices in how to display this molecule were critical creative acts. It seems to me that this defines work that should fall under copyright protection. The fact that all of these representations refer to the same underlying chemistry does not diminish in the least the creativity involved to create the last structure, and perhaps a total lack of creativity in producing the PubChem drawing.So it seems to me that one should be careful in the re-use of structure drawings. To me these “drawings” are not data, while the connection table, for example, is data.Steven