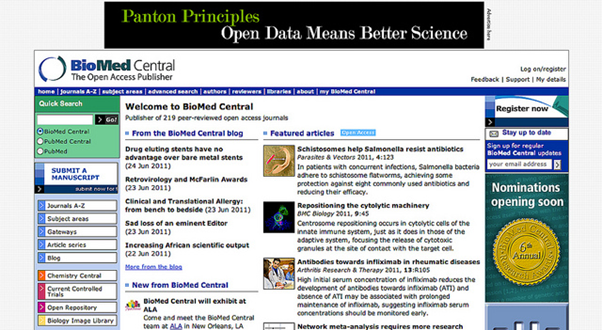
I am delighted that Biomed Central – an Open Access publisher (i.e. almost all its content is required to be full Open Access – CC-BY) has adopted and promoted the idea of Open Data. They have done me to honour twice of asking me to present their Open Data award (to authors who provide the most impressive use of Open Data). A little while ago they asked for simple phrases to go with the buttons and banners on the site and there were communal suggestions – and they chose this.
Simply – if your data are Open then the science is better. Why?
- Others can check your work
- Others can more easily evaluate your ideas and methodology. Science is about communication and Open Data helps scientific communication
- Others can re-use you data. You cannot guess what others may see in your “boring” data.
So this banner occurs in the add space of BMC. And the Open Data button occurs on many papers (i.e. the ones that have data specifically attached).
The Banner links back to the Panton Principles (http://pantonprinciples.org/) which was formulated by four of us and finally launched from the Panton Arms pub in Cambridge.
What’s Open Data?
http://www.biomedcentral.com/info/about/openaccess/#opendata
 By open data we mean that it is freely available on the public internet permitting any user to download, copy, analyse, re-process, pass them to software or use them for any other purpose without financial, legal, or technical barriers other than those inseparable from gaining access to the internet itself. We encourage the use of fully open formats wherever possible.
By open data we mean that it is freely available on the public internet permitting any user to download, copy, analyse, re-process, pass them to software or use them for any other purpose without financial, legal, or technical barriers other than those inseparable from gaining access to the internet itself. We encourage the use of fully open formats wherever possible.
Anyone is free:
- to copy, distribute, and display the work;
- to make derivative works;
- to make commercial use of the work;
Under the following conditions: Attribution
- the original author must be given credit;
- for any reuse or distribution, it must be made clear to others what the license terms of this work are;
- any of these conditions can be waived if the authors gives permission.
Statutory fair use and other rights are in no way affected by the above.
Simple. All you have to do is add the Open Data button and the licence to your data.
Open Data is coming and will be increasingly mandatory. Funders require grantees to have data management policies. Here’s any example of the steady progress (it’s about Open Access rather than data, but the two march together).
EPSRC joins other major funding agencies with new open access policy
 Earlier this month, the Engineering and Physical Sciences Research Council (EPSRC) announced its ‘Policy on Access to Research Outputs’, stating that all EPSRC-funded research must be published as open access (OA) documents. From September 1st 2011, all research must be published as either ‘Gold’ or ‘Green’ OA with the decision resting with the author.
Earlier this month, the Engineering and Physical Sciences Research Council (EPSRC) announced its ‘Policy on Access to Research Outputs’, stating that all EPSRC-funded research must be published as open access (OA) documents. From September 1st 2011, all research must be published as either ‘Gold’ or ‘Green’ OA with the decision resting with the author.
EPSRC joins numerous major funding agencies, such as the Wellcome Trust, NIH and NSF, in adopting an OA mandate. This policy has been adopted “in recognition of the need for increased availability and accessibility of publicly funded research findings.” Further information is available on their website.
This is an example of a meme that is spreading virally. Publishers, funders , authors will increasingly see he OPEN DATA button. If they don’t know what its, they’ll click and find out. Then they’ll think. And every time they see it they’ll think. And, sooner or later, they will realise that Open Data is the only way forward for data.