We had a welcoming session last week – see Egon Willighagen’s blog http://chem-bla-ics.blogspot.com/2010/10/are-these-organic-molecules-same.html where I casually asked what the relationship is between the following two molecules (I deprecate the use of the hatch in these pictures but that’s irrelevant here)
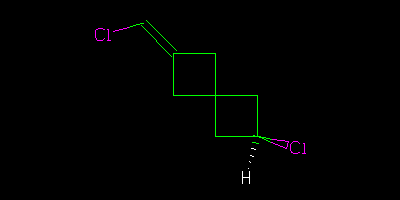
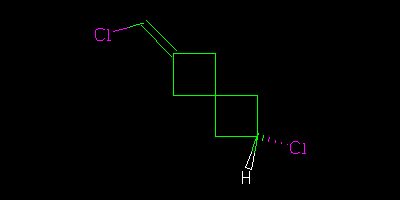
There is a definite answer, but think carefully
I will leave about a day, so we get a range of answers
I am a physicist, so I would say it is the same molecule in different conformations… just by rotating 180º over the double bond, I can get from one conformation to the other… the barrier must be high of course.
Maybe chemists would say that they are isomers.
Diastereomers. Assuming, that is, that the junction between the two four-membered rings is the same in both cases. Which bonds of one of the cyclobutanes are coming out of the paper? If that’s not consistent between the pictures then there’s not enough information to decide and they could be the same compound.
These are unusual cis/trans isomers, although the way they’re drawn makes it difficult to tell which is which. My instinct says the top is cis and the bottom trans.
You might expect me to give an unexpected answer, and that is to ask what the conformation of the 4-rings are? They are rarely planar, and tend to be buckled. Which would make the two compounds (which are of course cis/trans isomers) both chiral, each capable of existing as two enantiomers (although the enantiomerisation barrier is going to be tiny). If the 4-rings are planar, each molecule belongs to Cs symmetry, but again they are distinct isomers which will not interconvert. The systematic way of making such decisions is to use group theory. Anything belong to chiral groups (Cn, Dn etc) can have enantiomers.
I would say they are meso, Stereoisomers but not mirror images. Just by taking into consideration the way it is drawn (ignoring the stereochemistry of the spiro ring).
Stereoisomers but not mirror images – Diastereomers. (Corrected)
The drawing is very misleading – if the chlorine on the right is pointing up or down the chlorine on the left cannot be in the plane – it must point either up or down. The molecules are not chiral – they are just cis/trans isomers, no different than comparing cis and trans 1,2-dichloroethylene.
I agree with Jean-Claude. To be sure, the angles are not depicted as a chemist normally would in a molecular drawing, but assuming the usual bonding rules apply…the Cl is pointing up (perpendicular to the double bond) in the top molecule and pointing down in the bottom image. If you rotate one of them to superimpose the single bonded H and Cl, the Cl on the double bond will be on the opposite side. Which is cis and which is trans? Not so simple, and those terms may not be adequate.
Pingback: Unilever Centre for Molecular Informatics, Cambridge - More Molecules « petermr’s blog
Henry is definitely right about the bending of the cyclobutanes.
However, if we would ignore that fact for a moment to simplify the picture, the two spirocyclobutanes depicted could be described as cis and trans isomers.
Let’s assume that the cyclobutanes are orthogonal. The geometric relation of the substituents at the exomethylene carbon and that at the opposing cyclobutane methylene group would then be similar to the orientation found in a cumulene, i.e., the substituents are (roughly) in plane and allow for (E)- and (Z)-isomers.
I think both structures depict a mixture of cis/trans isomers.
One cyclobutane is in the plane of the alkene, the other cyclobutane is orthogonal to it and then the other chloride is orthogonal to that; this means the two chlorines in the same plane either cis or trans to each other. As the spiro centre is not defined by hashes/wedges, in either picture the cyclobutane/alkene portion can be attached to the other cyclobutane to form one isomer (for instance cis) or an isomer with this portion rotated by 180 degrees (for instance trans)
Pingback: Unilever Centre for Molecular Informatics, Cambridge - Miscellania « petermr’s blog