I was alerted last week by a blogospheric PhD student (worked with us for some time before going to Oxford) to the following story from Totally Synthetic (TotSynth).
NaH as an Oxidant – Liveblogging!
Even if you are not a scientist, please read on – it’s entertaining and informative. It deserves to be put in front of every young scientist as it shows the process of science as it should be done.
When I was at high school I read a popular and good chemistry paperback (Penguin) which highlighted the scientific method through a passage from Dorothy Sayers’ Strong poison where she describes in graphic and entertaining detail how A Marsh test for arsenic was carried out. The thread in the blogosphere captures competely the rigour, the attention to detail, the likelihood of false trails, the unexpected, the need for reference to authority and the need to question authority.
If I were teaching young chemists I would set them this as a real exercise. As a group, and in the lab. Give them a month. By the end of that month they would know far more about reactions, thermodynamics, spectra than they would get from formal lectures.
Moreover it highlights a real message of the evolving scientific web which is that what is said matters more than where it is published. For non-chemists I will interpret:
A group of scientists submitted a manuscript to The Journal Of The American Chemical Society. This is a well-known and high quality journal which is often used (naively) as a numeric metric of the value of a chemist (“how many JACS articles have they published?”). The ACS stresses the value of peer-review (as do I) and that its quality is low in Open Access journals (which I dispute). The published article (Reductive and Transition-Metal-Free: Oxidation of Secondary Alcohols by Sodium Hydride) is “advertised by the following graphical abstract (which I reproduce without permission as fair-use)
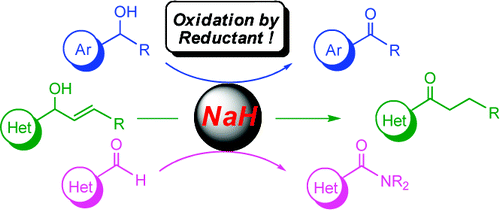
The potential utilities of the simplest hydride reductant sodium hydride (NaH) as an oxidation promoter have long been overlooked.
This claim is sensational in that if goes completely against received chemical knowledge. Any first year student, if given the top (blue) reaction would be expected to draw the arrow in the OTHER direction (right to left). They would certainly fail (part of) an exam if they wrote what the authors have claimed. So it’s not an obscure finding. If true it would mean that (free) energy would have to come from an unknown source. Not impossible, but extremely unlikely. On the order of cold fusion or Benveniste’s homeopathic water.
The claim apparently went through the reviewers and editors with little comment. But the blogosphere picked it up and Totally Synthetic decided to question the finding. You must read the blog. There’s a blending of careful attention and excitement – what IS the answer?
So I’m not going to give away the punchline. But I will say that the peer reviewing is closed so I cannot absolutely comment on whether the paper should have been accepted. Currently I regard the paper as an outstanding example of junk science published in a journal which prides itself on selling high-quality science. But I haven’t read the paper (as it’s closed access and will cost me 30 GBP for 2 days only). So my mind always remains slightly open.
This should convince any sceptic that the blogosphere is an essential part of current science.
I was alerted to the paper by readers of my blog, who noticed its controversial abstract almost as soon as it appeared online,’ says Docherty. ‘A quick inspection of Wang’s results astounded me, as he seemed to suggest that black was apparently now white; most curiously, his postulated mechanism only accounted for half of his results. Most provocative papers in organic chemistry take some time and resources to verify, but Wang’s chemistry seemed very amenable to a quick test reaction. It only took a few minutes to set up his chemistry in my fume-hood, and a similarly short amount of time to analyse the results. As I was writing about this on my blog, my readers did likewise, each using slightly different materials and conditions, allowing a very quick “scoping” of the chemistry.’
Some oxidation of alcohols was observed in most cases, but a consensus was rapidly reached that an oxidising contaminant was making its way into the reaction, be it oxygen from the air adsorbed to the NaH, or traces of sodium peroxide or hydroxide or some other trace contaminant. When stringent steps were taken to ensure absolutely that no air could enter the reaction system, no oxidation was seen.
Glad you decided to mention this Peter. 🙂 This kind of publishing is total trash. If there is sufficient supporting evidence and control experiments to support these findings then they should be included as part of the publication – either in the main text or (freely available) supporting information.
No self-respecting organic chemist should be happy to accept the findings in this paper. If anything, this should serve as a caution to those people who often treat a literature preparation as gospel when in fact it should always be treated as highly suspicious.
Thanks Justin,
I would have published immediately under normal circumstances and hadn’t been coding. I feel very strongly for young people like you and colleagues. It’s a hard slog, nature is a hard task mistress and most science does not work out, and you should all feel justly proud when you finally manage to get publishable results. I don’t know how this got through. This is worse than Wiley’s creationist proteomics because it’s so in-your-face. No-one who knows any chemistry could possibly fail to react with shock at the title.
The blogosphere including you emerge with enormous credit
Cold fusion is unlikely, but it has been replicated in hundreds of major labs, in thousands of experiments. I have a collection of 1,200 peer-reviewed papers on this subject, copied from the library at Los Alamos. See:
http://lenr-canr.org
So, just because something is unlikely, that does not make it false.
Thanks,
You are right to chide me – I was sloppy in my language. I was referring to the Fleischman-Pons affair, not cold fusion in general.
It is not *impossible* that the current reaction happened assuming it can find a source of free energy. That might come from an unknown source (in this case probably oxygen so uninteresting) or a displacement of the reaction (e.g. by complete removal of hydrogen gas). Or it might be some form of electromagnetic radiation or …
But because it is unlikely means that the peer-review has to be even tighter than normal. Which is what Nature and Randi did for Benveniste… (see http://www.bbc.co.uk/science/horizon/2002/homeopathy.shtml while I find the Nature reference).
It’s a little inaccurate to call it “junk science.” The reactions work, they’ve been verified by several people in the comments section at TS as well as by Paul himself. The problem is in the interpretation, as it’s adventitious oxygen that’s doing the oxidation. Yes, it’s poor refereeing, but more like “junk interpretation” rather than “junk science”. Also, oxidations with molecular oxygen are a current topic and this will likely lead to some interesting new examples of that now that so much of the community has been exposed to this old but obscure reactivity.
Thanks Tok,
In a vacuum you are correct, but…
… The phrase “Junk Science” was coined by PRISM of which the ACS was a founder member (though much of this was based on unpublished knowledge). The purpose of PRISM was to denigrate the Open Access movement as providing Junk Science due to lack of peer review. This is the type of science which PRISM members would undoubtedly have used as evidence (though they did not, apparently, find any). Therefore when it happens in their journals it is not unreasonable to use the phrase that they coined.
If the paper had been called “oxidation due to adventitious oxygen in insufficiently degassed experiments” it would have been a reasonably accurate title. Whether it would have been worthy of publication either due to methodology or novelty I doubt.
The discussion in the paper is based on the interpretation of Hydride as an oxidising agent. The discussion on mechanism contains the phrase:
This strongly suggest that the authors do not accept the mainstream view of oxidation and reduction and their image (which is not copyable technically from the paper) does not make sense in mainstream chemistry
You wrote:
“You are right to chide me – I was sloppy in my language. I was referring to the Fleischman-Pons affair, not cold fusion in general.”
There was no “affair.” They published a paper in J. Electroanal. Chem., and when it was in print they held a press conference. This is a perfectly normal thing to do. Plasma fusion researchers usually call a press conference and announce success immediately after a major test, sometimes months before they publish a paper. Unfortunately, with pre-Internet technology it was difficult to get a copy for a few days. You can watch the press conference on YouTube and you will see that it was sober, accurate and all the claims they made were replicated within a year by roughly 100 groups around the world. (~20 groups failed to replicate, for reasons that are obvious in retrospect.)
The paper was written in hurry so it was not well written compared to most of Fleischmann’s work. The University of Utah pushed them to go public because of intellectual property concerns. They had been working on it since the early ’80s and Fleischmann wanted to keep the research secret for another 3 to 5 years.
Fleischmann had been thinking about the subject since the late 1940s. This field is older than that. The first observations of cold fusion were reported in 1927, even before the discovery of deuterium. In the 1980s Mizuno and others observed charged particles and other anomalies in the D-Pd system. Fleischmann and Pons were the first to make solid, replicated observations, and the first to discover that the number of neutrons per joule of heat is about 11 million times lower than with plasma fusion. That was very surprising, and it is the reason many plasma fusion researchers rejected the claims in 1989, and still do.
Tok: There is more to science than getting the stated outcome. The forming and testing of hypotheses is central, and that is where this paper falls short. Their tests were insufficiently rigorous, and their hypothesis has been falsified.
Furthermore, their experimental procedure states that the reaction was performed under a nitrogen atmosphere. I challenge you to reproduce their results under rigorously pure nitrogen.
Pingback: Notes on Research Papers » No one added their test on oxidative NaH to ResearchBlogging.org?
The reactions work, they’ve been verified by several people in the comments section at TS as well as by Paul himself. The problem is in the interpretation, as it’s adventitious oxygen that’s doing the oxidation. Yes, it’s poor refereeing, but more like “junk interpretation” rather than “junk science”. Also, oxidations with molecular oxygen are a current topic and this will likely lead to some interesting new examples of that now that so much of the community has been exposed to this old but obscure reactivity.